Shipp Lab ResearchOur translational research group focuses on the pathogenesis and treatment of aggressive B-cell lymphomas, including the most common lymphoid malignancy, diffuse large B-cell lymphoma (DLBCL), large B-cell lymphoma (LBCL) subtypes and classical Hodgkin lymphoma (cHL). We have developed genome-wide approaches to define the unique molecular signatures of specific lymphoid malignancies and more rational therapeutic targets.
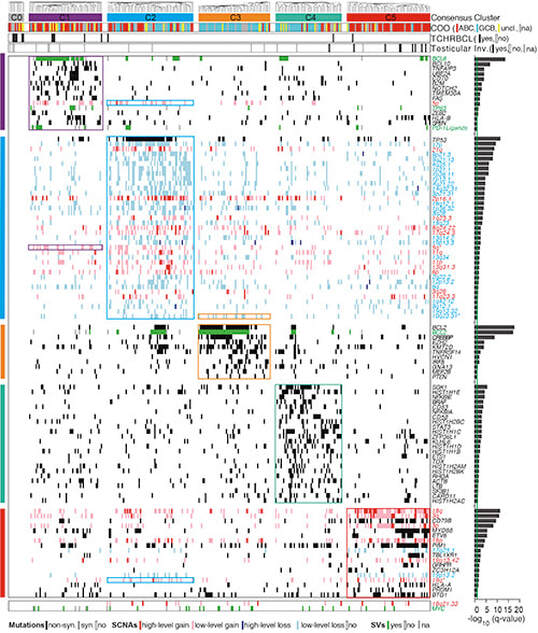
Transcriptionally defined consensus clusters and recurrent copy number alterations in DLBCL. We previously utilized a large series of newly diagnosed DLBCLs, transcriptional profiling, multiple clustering methods and gene set enrichment analysis to identify three discrete subsets of DLBCLs – “Oxidative Phosphorylation (OxPhos)”, B-cell Receptor/Proliferation (BCR)” and “Host Response” (HR). HR tumors, which were largely defined by their inflammatory/immune cell infiltrate, primarily included DLBCLs identified as T-cell/histiocyte-rich large B-cell lymphomas (WHO Classification). Thereafter, we characterized pathogenetic mechanisms and associated rational therapeutic targets in the BCR and OxPhos DLBCLs. We found that BCR-type DLBCLs were reliant upon B-cell receptor signaling and uniquely sensitive to targeted inhibition of critical BCR survival pathway components. In additional collaborative studies with the Danial group, we defined distinct fuel utilization pathways in BCR DLBCLs, which primarily rely on aerobic glycolysis, and OxPhos DLBCLs, which largely depend upon fatty acid oxidation. OxPhos DLBCLs are selectively sensitive to inhibition of fatty acid oxidation suggesting that the recently defined subtype-specific metabolic dependencies may lead to targeted approaches to treatment. In more recent genetic analyses of DLBCL, we comprehensively defined recurrent copy number alterations and the associated driver genes and identified an outcome-associated and targetable pattern of p53 and cell cycle deregulation in addition to recurrent somatic mutations. LBCL subtypes, cHL and the 9p24.1/PD-L1/PD-L2 amplicon. In addition to identifying hallmark genetic features of DLBCL, we have characterized the signatures of discrete LBCL subtypes such as primary mediastinal large cell lymphoma (MLBCL). The unique molecular signature of MLBCL revealed unanticipated links between this LBCL subtype and cHL, including a shared signaling and survival pathway (NFkB) and a genetic basis of immune evasion – 9p24.1/PD-L1/PD-L2 copy number alterations and associated over-expression of the PD-1 ligands. In almost all cases, the 9p24.1 amplicon extends to include JAK2, of note because the associated JAK/STAT signaling further upregulates PD-1 ligand expression Genetically driven PD-1 signaling and PD-1 blockade in cHL and LBCL subtypes. The identification of genetically driven PD-1 mediated immune evasion led to the development of multicenter clinical trials of PD-1 blockade in cHL and MLBCL. We were instrumental in the development and conduct of pilot studies of PD-1 blockade in relapsed/refractory cHL. The 66- 87% response rates and long-lasting remissions led the FDA to confer breakthrough status for PD-1 blocking antibodies in relapsed/refractory cHL. An international registration trial of PD-1 blockade (nivolumab) in relapsed/refractory cHL confirmed the previously reported activity and resulted in FDA approval on May 17, 2016. Pilot studies of PD-1 blockade (pembrolizumab) in relapsed/refractory MLBCL revealed a 40% response rate with durable remissions, prompting the development of a recently opened national/ international confirmatory phase II trial. In recent studies, we identified recurrent 9p24.1/PD-L1/PD-L2 copy number alterations in 2 additional poor-prognosis LBCLs, primary central nervous system lymphoma (PCNSL) and primary testicular lymphoma (PTL) (see News). The recurrent 9p24.1 alterations and the clinical activity of PD-1 blockade (nivolumab) in a small pilot series of patients with PCNSL led to the development of an international phase II study of PD-1 blockade in relapsed/refractory PCNSL and PTL that will open in mid 2016. Additional immune evasion mechanisms in lymphoid malignancies. We have also characterized additional immune evasion mechanisms in cHL in which small numbers of malignant Reed-Sternberg (RS) cells reside within an extensive inflammatory/immune cell infiltrate. We found that HL cell lines and primary HLs overexpress galectin-1 (Gal1), a carbohydrate-binding lectin that selectively induces the apoptosis of cytotoxic T cells and Th1 cells, skews the balance toward a Th2-type cytokine profile and favors the expansion/retention of Treg cells. These studies directly implicated RS cell Gal1 in the development and maintenance of immunosuppressive Th2/Treg-skewed microenvironment in cHL. In recent collaborative studies with the Rabinovich group, we also defined a role for Gal1 in VEGF-dependent tumor angiogenesis. Because Gal1 represents a novel therapeutic target for restoring immune surveillance in cHL and additional Gal1+ malignancies, we have developed neutralizing Gal1 monoclonal antibodies that are slated for clinical development. |
Publication of our analysis of 9p24.1/CD274(PD-L1)/ PDCD1LG2(PD-L2) alterations in a clinically annotated series of classical Hodgkin lymphomas (cHL) (J Clin Oncol 2016;34(23):2690-7). Ninety-seven percent of all evaluated cHLs had concordant alterations of the PD-L1 and PD-L2 loci, including copy gain in 56% of patients and amplification in 36% of patients. Progression-free survival was significantly shorter for patients with 9p24.1 amplification who were also more likely to have advanced stage disease. In cHL, the near-uniform alterations of the PD-L1/PD-L2 loci likely explain the activity of PD-1 blockade in this disease.FDA approval for PD-1 blockade (nivolumab therapy) in relapsed/ refractory cHL in May, 2016. The approval was based on data from the pilot study (N Engl J Med 2015;372(4):311-9) and subsequent registration trial (Lancet Oncology 2016 July 20. Epub ahead of print), including a response rate of 66-87% in heavily pre-treated patients.
Publication of our comprehensive genomic analysis of primary central nervous system lymphoma (PCNSL) and primary testicular lymphoma (PTL) (Blood 2016;127(7):869-81). To identify targetable genetic features of PCNSL and PTL, we characterized their recurrent somatic mutations, chromosomal rearrangements, copy number alterations (CNAs) and associated driver genes and compared these comprehensive genetic signatures to those of diffuse LBCL and primary mediastinal large B-cell lymphoma (PMBL). PCNSLs and PTLs exhibit near-uniform, often biallelic, CDKN2A loss with rare TP53 mutations. PCNSLs and PTLs also utilize multiple genetic mechanisms to target key genes and pathways and exhibit near-uniform oncogenic Toll-like receptor signaling due to MYD88 mutation and / or NFKBIZ amplification, frequent concurrent B-cell receptor pathway activation and deregulation of BCL6. Of great interest, PCNSLs and PTLs also have frequent 9p24.1/PD-L1/PD-L2 CNAs and additional translocations of these loci, structural bases of immune evasion that are shared with PMBL. A clinical trial of PD-1 blockade (nivolumab therapy) in relapsed/refractory PCNSL and PTL is scheduled to open in early fall, 2016.
|
- HOME
- RESEARCH/NEWS
- PUBLICATIONS
-
PERSONNEL
- Lab Personnel
-
Groups
>
- Administrative Team
- Analyses of Immune Evasion Mechanisms and Signaling Pathways in Large B-Cell Lymphomas
- Comprehensive Molecular Signatures of Lymphoid Malignancies
- DNA Damage Repair Pathways in Lymphoid Malignancies
- Genetic Bases of Immune Evasion in Hodgkin Lymphoma
- Molecular Signatures of Lymphoid Malignancies
- Molecular Signatures and Associated Therapeutic Targets
- Multiparametric Analyses of Essential Signaling Pathways
- Physiologic and Oncogenic B-Cell Receptor Signaling
- COLLABORATORS
- FUNDING
- CONTACT
HOME | RESEARCH/NEWS | PUBLICATIONS | PERSONNEL | COLLABORATORS | FUNDING | CONTACT
©2021 Shipp Lab at Dana-Farber Cancer Institute
©2021 Shipp Lab at Dana-Farber Cancer Institute
- HOME
- RESEARCH/NEWS
- PUBLICATIONS
-
PERSONNEL
- Lab Personnel
-
Groups
>
- Administrative Team
- Analyses of Immune Evasion Mechanisms and Signaling Pathways in Large B-Cell Lymphomas
- Comprehensive Molecular Signatures of Lymphoid Malignancies
- DNA Damage Repair Pathways in Lymphoid Malignancies
- Genetic Bases of Immune Evasion in Hodgkin Lymphoma
- Molecular Signatures of Lymphoid Malignancies
- Molecular Signatures and Associated Therapeutic Targets
- Multiparametric Analyses of Essential Signaling Pathways
- Physiologic and Oncogenic B-Cell Receptor Signaling
- COLLABORATORS
- FUNDING
- CONTACT